All
News Articles
Publications
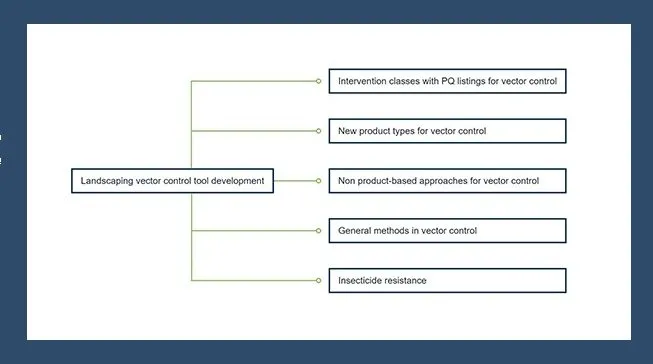
Recommended updates to the WHO susceptibility bioassay method based on interpretation and application of the method over time

The World Health Organization developed the “WHO susceptibility bioassay” method in the 1960s to monitor insecticide resistance in mosquito populations. The method has evolved in the 60+ years since it was first developed, leading to questions of how varied interpretation and application of the method over time may affect the accuracy of bioassay results.
A literature review was conducted that involved reviewing the most current published test procedures and examining published literature that cites the WHO susceptibility bioassay method to understand how the method is being used in practice. This literature review revealed inconsistencies both in how the method is interpreted and how it is applied.
Following the literature review, the researchers further explored how varying interpretations of certain parameters and varying execution of the test procedures could impact bioassay results. Parameters assessed for their impact on bioassay results included: covering or uncovering of the tube end during exposure, the number of mosquitoes per test unit, and mosquito age. Results showed that using fewer than the recommended 15–30 mosquitoes per test unit significantly reduced mortality, covering the exposure tube had no significant effect, and using mosquitoes older than 2–5 days old increased mortality, particularly in the resistant strain.
Based on these results, the researchers recommended updates to the bioassay method focused on (1) tightening these parameters to help prevent inaccurate measures of insecticide resistance and (2) implementing consistent practices to generate more robust data. The recommendations include:
- Better reporting of the conditions that a bioassay is carried out under (holding/exposure temperature, holding/exposure humidity, and other conditions).
- All bioassay testing should be carried out with WHO tubes positioned vertically (as detailed in the test procedures).
- A minimum of 15 and a maximum of 30 mosquitoes should be tested per test unit.
- Use of a characterized reference strain alongside bioassay testing of field strains is recommended.
- The practice of using cardboard discs to cover exposure tubes does not appear to be necessary but should be continued until more comprehensive data is generated on the effect of light intensity on bioassay outcomes.
- Historical updates and discussions of the test procedures should be clearly marked and should be linked to the most recent version of the test procedures.
Access to the publication “Reviewing the WHO Tube Bioassay Methodology: Accurate Method Reporting and Numbers of Mosquitoes Are Key to Producing Robust Results”. Giorgio Praulins, Daniel P. McDermott, Angus Spiers, Rosemary Susan Lees.