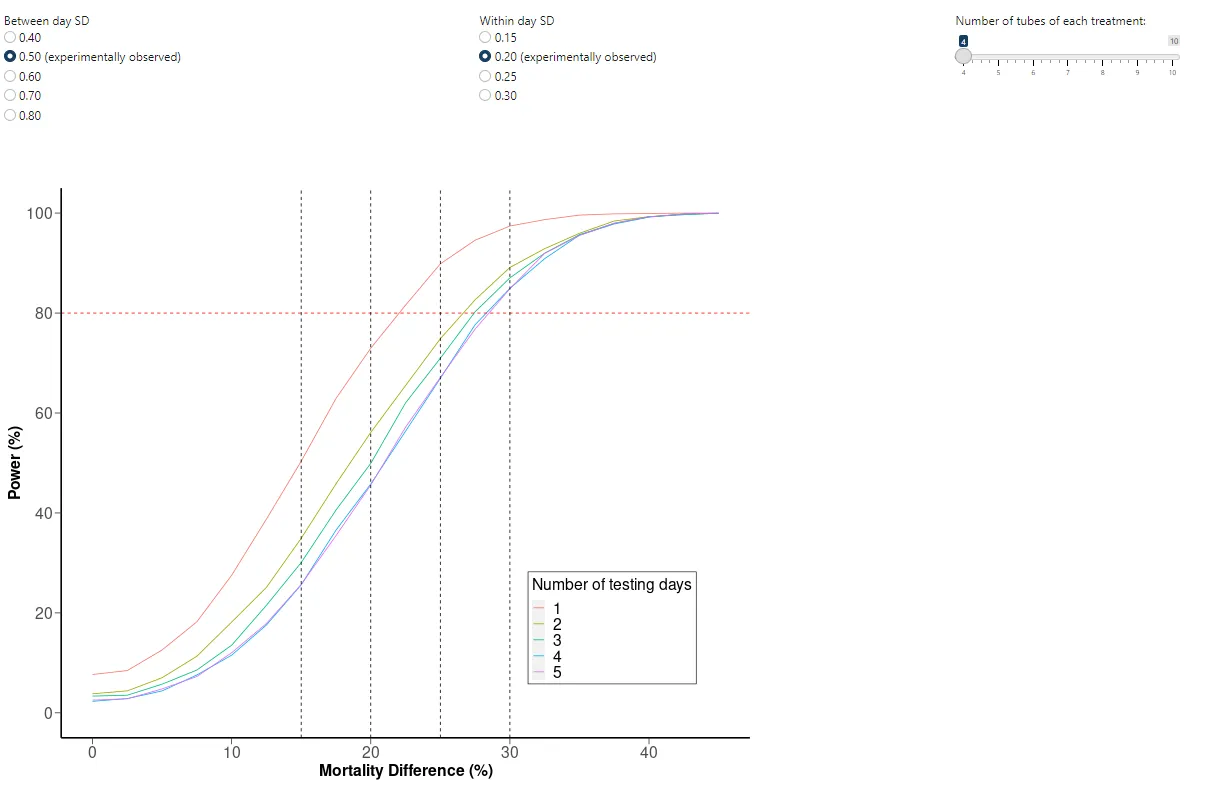
All
News Articles
GLP Site Sustainability Workshop

i2i recently held a workshop in Liverpool to support trial sites on sustaining their business.
The workshop was arranged in response to an i2i survey which reported earlier this year and identified a number of issues that sites wanted support on how to maintain their business operations once they have become GLP (Good Laboratory Practice) compliant.
A number of sites across Africa, Asia and Latin America are being supported by IVCC and WHO to achieve Good Laboratory Practice (GLP) certification as part of the change in the WHO vector control product evaluation process to the Prequalification (PQ) system.
PQ stipulates GLP as the quality standard for all data submitted, and so the sites are being supported to enhance their systems and infrastructure.
The 3 days in Liverpool focussed on a number of issues including costing, pricing and client relationship building, and sites were encouraged to enhance their business plans in light of the themes covered.
The event was attended by sites from Tanzania, Benin, Cote D’Ivoire, Burkina Faso and China. WHO are planning a similar workshop for the remainder of the Asian sites in February 2019, in New Dehli.